Abnormal Molar Masses
`color{green}("For Dissociation :")` We know that ionic compounds when dissolved in water dissociate into cations and anions.
● For example, if we dissolve one mole of `KCl` (74.5 g) in water, we expect one mole each of `K^+` and `Cl^-` ions to be released in the solution. If this happens, there would be two moles of particles in the solution. So, one mole of `KCl` in one kg of water would be expected to increase the boiling point by `2xx0.52K = 1.04 K`.
● If we did not know about the degree of dissociation, we could be led to conclude that the mass of 2 mol particles is 74.5 g and the mass of one mole of `KCl` would be 37.25 g. This conclude that, when there is dissociation of solute into ions, the experimentally determined molar mass is always lower than the true value.
`color{green}("For Association :")`
● Molecules of ethanoic acid (acetic acid) dimerise in benzene due to hydrogen bonding. This normally happens in solvents of low dielectric constant. In this case the number of particles is reduced due to dimerisation. Association of molecules is depicted as follows :
● If all the molecules of ethanoic acid associate in benzene, then `ΔT_b` or `ΔT_f` for ethanoic acid will be half of the normal value.
● Therefore, the molar mass calculated on the basis of this `ΔT_b` or `ΔT_f` will be twice the expected value.
`color{green}("Abnormal Mass") :` Such a molar mass that is either lower or higher than the expected or normal value is called as abnormal molar mass.
In 1880 van’t Hoff introduced a factor `i`, known as the van’t Hoff factor, to account for the extent of dissociation or association. This factor `i` is defined as :
`color{green}(i =text{Normal molar mass}/text{Abnormal molar mass} )`
` = color{green}(text(Observed colligative property)/text(Calculated colligative property))`
`color{green}(i = text(Total number of moles of particles after association/dissociation)/text(Number of moles of particles before association/dissociation))`
Here, abnormal molar mass = experimentally determined molar mass
Calculated colligative properties are obtained by assuming that the non-volatile solute is neither associated nor dissociated.
● In case of association, value of `i` is less than unity
● In case of dissociation, it is greater than unity.
`=>` For example, the value of `i` for aqueous `KCl` solution is close to `2`, while the value for ethanoic acid in benzene is nearly `0.5`.
`=>` Inclusion of van’t Hoff factor modifies the equations for colligative properties as follows :
Relative lowering of vapour pressure of solvent. `(p_1^0 - p_1)/(p_1^0) = i n_2/n_1`
Elevation of Boiling point, `DeltaT_b = i K_b m`
Depression of Freezing point, `DeltaT_f = i K_f m`
Osmotic pressure of solution, `pi = i n_2RT//V`
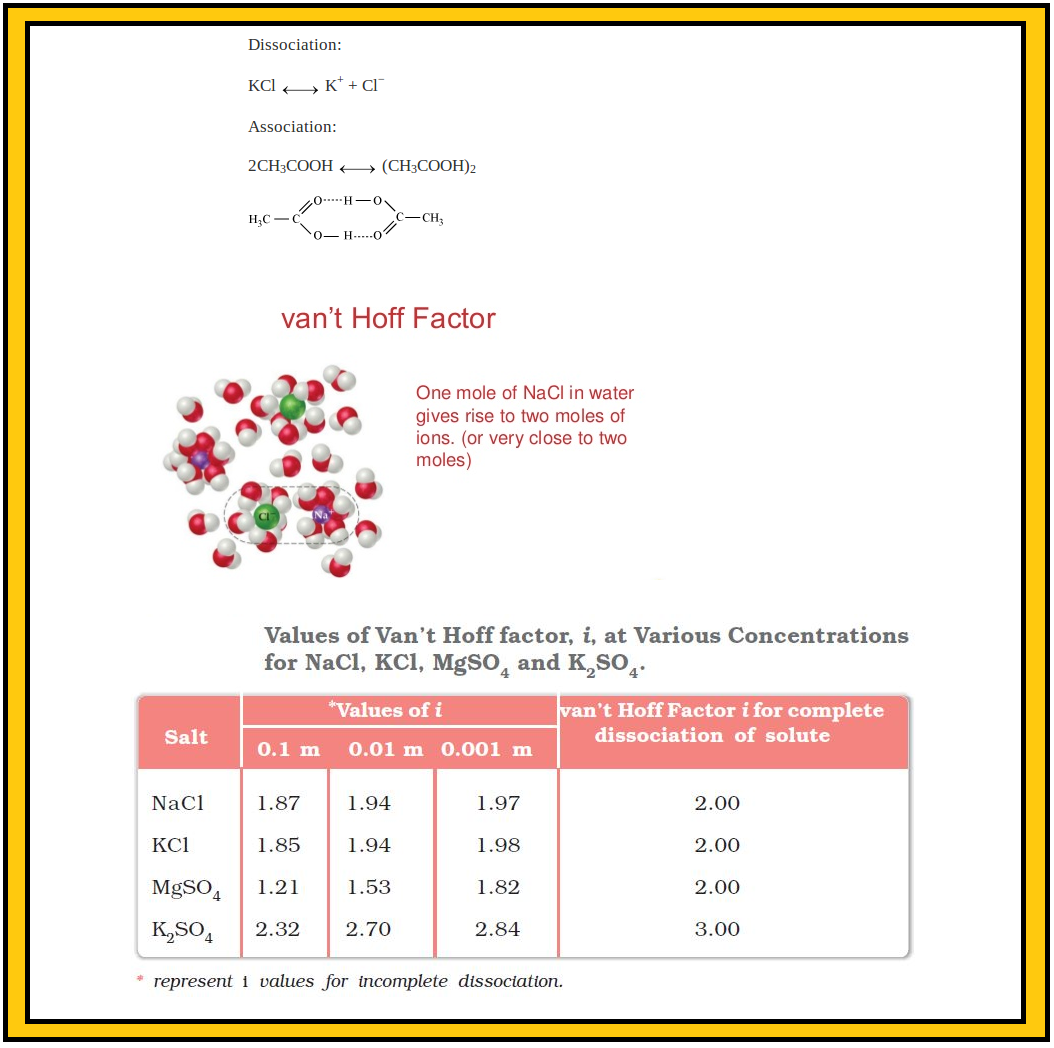
Table 2.4 depicts values of the factor, `i` for several strong electrolytes. For `KCl, NaCl` and `MgSO_4, i` approach 2 as the solution becomes very dilute. As expected, the value of `i` gets close to 3 for `K_2SO_4`.
● For example, if we dissolve one mole of `KCl` (74.5 g) in water, we expect one mole each of `K^+` and `Cl^-` ions to be released in the solution. If this happens, there would be two moles of particles in the solution. So, one mole of `KCl` in one kg of water would be expected to increase the boiling point by `2xx0.52K = 1.04 K`.
● If we did not know about the degree of dissociation, we could be led to conclude that the mass of 2 mol particles is 74.5 g and the mass of one mole of `KCl` would be 37.25 g. This conclude that, when there is dissociation of solute into ions, the experimentally determined molar mass is always lower than the true value.
`color{green}("For Association :")`
● Molecules of ethanoic acid (acetic acid) dimerise in benzene due to hydrogen bonding. This normally happens in solvents of low dielectric constant. In this case the number of particles is reduced due to dimerisation. Association of molecules is depicted as follows :
● If all the molecules of ethanoic acid associate in benzene, then `ΔT_b` or `ΔT_f` for ethanoic acid will be half of the normal value.
● Therefore, the molar mass calculated on the basis of this `ΔT_b` or `ΔT_f` will be twice the expected value.
`color{green}("Abnormal Mass") :` Such a molar mass that is either lower or higher than the expected or normal value is called as abnormal molar mass.
In 1880 van’t Hoff introduced a factor `i`, known as the van’t Hoff factor, to account for the extent of dissociation or association. This factor `i` is defined as :
`color{green}(i =text{Normal molar mass}/text{Abnormal molar mass} )`
` = color{green}(text(Observed colligative property)/text(Calculated colligative property))`
`color{green}(i = text(Total number of moles of particles after association/dissociation)/text(Number of moles of particles before association/dissociation))`
Here, abnormal molar mass = experimentally determined molar mass
Calculated colligative properties are obtained by assuming that the non-volatile solute is neither associated nor dissociated.
● In case of association, value of `i` is less than unity
● In case of dissociation, it is greater than unity.
`=>` For example, the value of `i` for aqueous `KCl` solution is close to `2`, while the value for ethanoic acid in benzene is nearly `0.5`.
`=>` Inclusion of van’t Hoff factor modifies the equations for colligative properties as follows :
Relative lowering of vapour pressure of solvent. `(p_1^0 - p_1)/(p_1^0) = i n_2/n_1`
Elevation of Boiling point, `DeltaT_b = i K_b m`
Depression of Freezing point, `DeltaT_f = i K_f m`
Osmotic pressure of solution, `pi = i n_2RT//V`
Table 2.4 depicts values of the factor, `i` for several strong electrolytes. For `KCl, NaCl` and `MgSO_4, i` approach 2 as the solution becomes very dilute. As expected, the value of `i` gets close to 3 for `K_2SO_4`.



